Introduction: The Journey of Discovery
In the realm of science, discoveries often emerge serendipitously. The captivating narrative of zeolites, starting with their whimsical name “boiling stones,” offers an enchanting insight into the evolution of materials science. This is particularly evident in the context of the award-winning research project titled “Synthesis and Application of Ordered Mesoporous Polymers and Carbon Materials,” led by Professor Zhao Dongyuan from Fudan University, which garnered the prestigious National Natural Science Award in 2020.
A Curious Discovery
In 1756, Swedish mineralogist Alex Cronstedt made a remarkable observation while heating a mineral with a flame. He noted that the mineral responded by bubbling, expanding, and releasing steam, leading him to label it as “zeolite,” a term synonymous with “boiling stone.” However, Cronstedt was unaware of the underlying principles governing this phenomenon. It wasn’t until over a century later that researchers established the reversible nature of the water loss observed by Cronstedt, showcasing zeolites as materials capable of cyclic dehydration and rehydration.
Unraveling the Complexity of Zeolites
Modern scientific inquiry has classified zeolites as a subset of porous crystalline materials composed of aluminosilicates, featuring pore sizes ranging from 0.3 nm to 1.5 nm. The commercial potential of zeolites was recognized in the early 20th century when they were utilized for softening hard water by removing excessive calcium and magnesium ions, a practice that continues to this day.
The discovery in 1925 of zeolites’ capability to separate gas molecules based on size initiated a new chapter in material science, leading to the concept of “molecular sieves.” British chemist Richard Barrer significantly advanced this field by synthesizing new types of zeolite molecules under controlled conditions, paving the way for the synthetic production of zeolite structures that do not occur in nature.
The Era of Synthetic Zeolites
As the 1950s approached, the industrial utility of zeolitic molecular sieves became increasingly apparent. Researchers like Robert Milton from Union Carbide Corporation made pioneering strides, developing types A, B, and X zeolites for commercial applications. These synthetic zeolites have remained vital in chemical processes, shaping the landscape of catalysis and adsorption.
Today, the International Zeolite Association recognizes over 247 distinct zeolite framework types, illustrating the extensive research undertaken in this field.
The Evolving Landscape of Mesoporous Materials
While zeolite research flourished, the limitations in pore size prompted the exploration of mesoporous materials. The term “mesoporous” refers to materials with pore sizes between 2 nm and 50 nm. In 1991, a breakthrough occurred when researchers at Mobil Oil Corporation published their synthesis of the first ordered mesoporous silica, MCM-41. Utilizing surfactants as templates, this innovative approach enabled the formation of highly ordered hexagonal pore structures, distinguishing mesoporous materials from classical zeolites.
Despite MCM-41’s success, challenges such as thin pore walls and poor thermal stability remained. This motivated the search for improved synthesis techniques, leading to the creation of the Santa Barbara Amorphous-15 (SBA-15) in 1998, which demonstrated enhanced stability and control over pore sizes.
Innovative Approaches to Synthesis
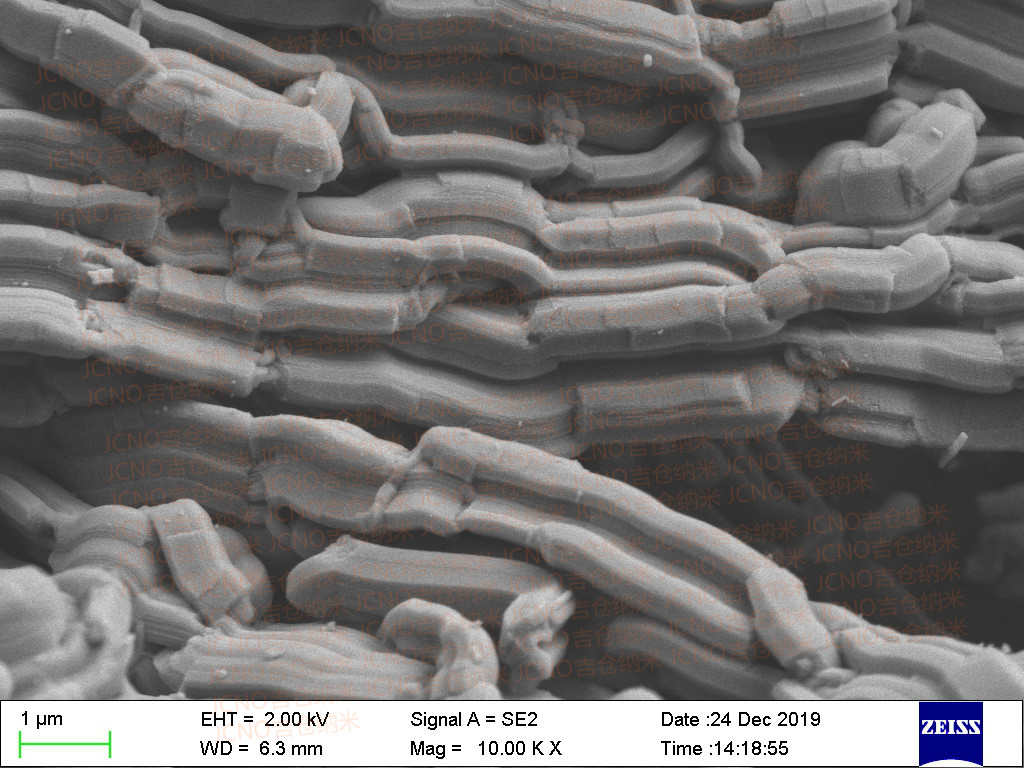
As research continued, scientists explored various methodologies for preparing ordered mesoporous materials. The hard template approach, wherein existing stable mesoporous structures are used as molds, has led to the successful synthesis of a variety of new materials, including ordered mesoporous carbon.
The advent of mesoporous carbon, pioneered by researchers at KAIST, opened a new dimension in materials science. The ability to manipulate the porous structure of carbon led to significant advancements in energy storage and catalysis.
Expanding Horizons: Non-Silica Mesoporous Materials
In the pursuit of developing non-silica mesoporous materials, Zhao Dongyuan’s research team introduced groundbreaking concepts, including the “acid-base reaction pairing” method, which allowed for the controlled synthesis of ordered mesoporous metal oxides and phosphates. Their innovative approaches have transformed the understanding and application of mesoporous materials in various fields.
The Dawn of Organic Mesoporous Materials
The evolution of mesoporous materials has now embraced organic structures as well. In 2005, Zhao’s team suggested an innovative approach involving the self-assembly of phenolic resins, resulting in stable and highly ordered organic mesostructures. This laid the groundwork for the synthesis of mesoporous organics and carbons, signifying a prominent shift in the landscape of porous materials.
Present and Future: A Bright Horizon for Mesoporous Materials
Today, the field of mesoporous materials is brimming with potential. The research spearheaded by Zhao Dongyuan and his team has not only revealed the intricate assembly methods for these materials but also expanded their applicability in numerous sectors, including renewable energy and biomedicine.
As we reflect on the journey from the discovery of zeolites to the advanced synthesis of ordered mesoporous materials, it becomes clear that the explorations in porous materials chemistry remain as dynamic and promising as ever. The innovative pathways generated by continuous research will undoubtedly lead to further breakthroughs, answering long-standing challenges and unlocking new possibilities for the future.
Conclusion: A Legacy of Innovation
As we celebrate over 250 years of zeolite study and 30 years of ordered mesoporous material development, it is evident that the quest for knowledge in porous materials chemistry will persist, enriching our understanding and applications in diverse fields. The pursuit of scientific discovery, driven by curiosity and innovation, will continue to illuminate the future of materials science.










































Discussion about this post