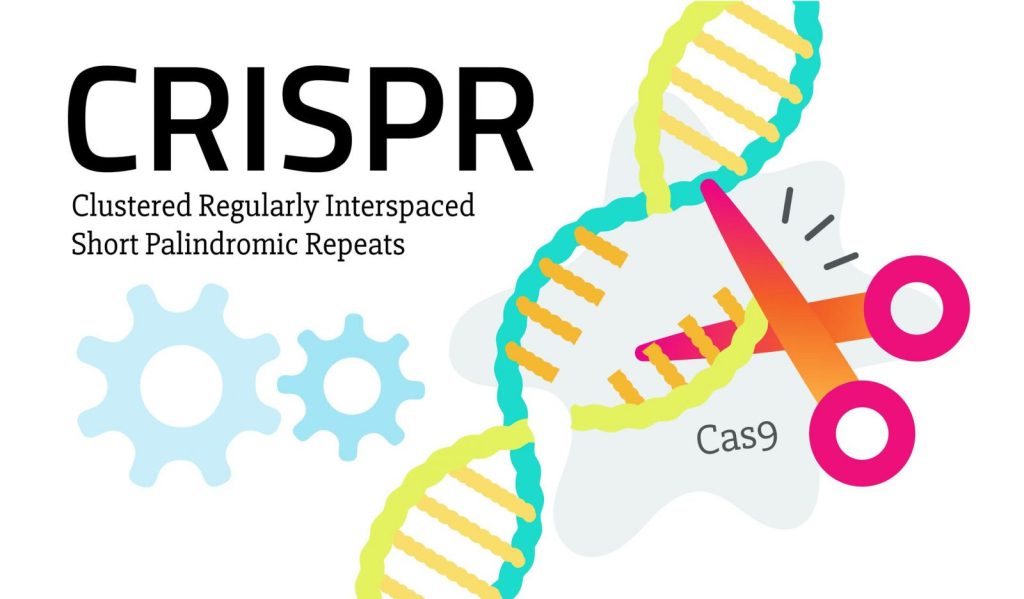
Pioneering Repeated Dosing with CRISPR/Cas9 Gene Editing
In the expansive frontier of genetic medicine, Intellia Therapeutics strides forward, manifesting the first clinical data to demonstrate the potential for repeated dosing with an in vivo CRISPR/Cas9 gene-editing therapy. This groundbreaking revelation not only reinforces the concept of in vivo gene editing but also indicates its potential applicability in treating a gamut of diseases where multiple dosing could be requisite.
The Clinical Trailblazers
On June 25, 2024, Intellia, founded by Nobel Laureate Jennifer Doudna, disclosed the latest clinical data concerning their CRISPR/Cas9 gene-editing therapy, NTLA-2001. Three patients with Transthyretin (ATTR) Amyloidosis, previously administered the lowest dose in a Phase I dose-escalation study, saw a median reduction of 90% in serum Transthyretin (TTR) protein levels following a successive 55 mg NTLA-2001 treatment.
The Battle Against ATTR Amyloidosis
Hereditary Transthyretin (ATTRv) Amyloidosis, a rare yet fatal condition, emerges from genetic mutations leading to abnormal protein production, accumulating and inflicting damage on vital organs such as the heart and nervous system. While curative therapy remains elusive with, currently available medications only slowing the misfolded TTR accumulation, NTLA-2001 offers a ray of hope by inactivating the TTR gene, thereby reducing TTR protein levels in the serum.
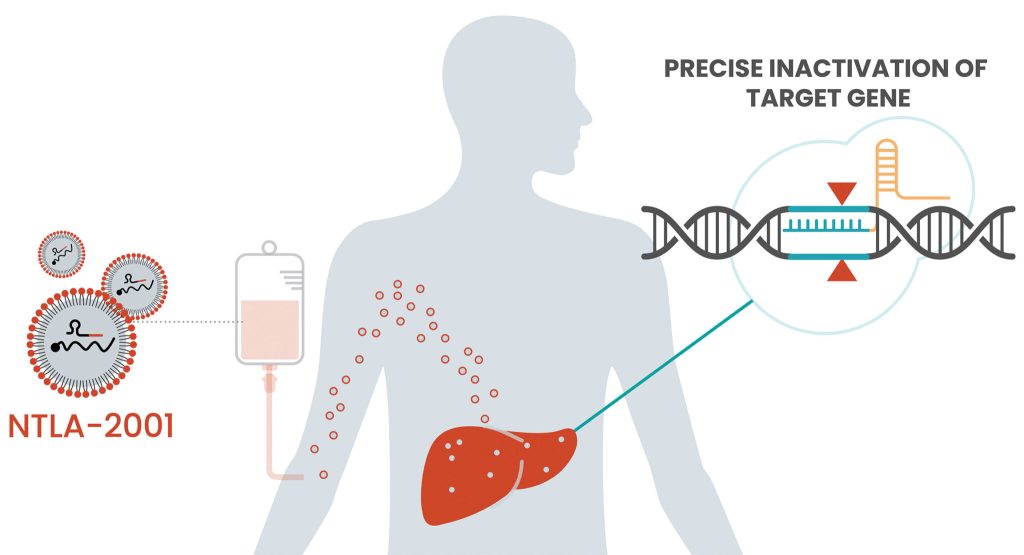
Strategic Alliances for Innovation
Intellia announced a key collaboration with Regeneron Pharmaceuticals on June 1, 2020, driving forward the development and commercialization of NTLA-2001, with Regeneron shouldering 25% of the costs in exchange for an equal share in profits.
Clinical Data Insights
Initial results from the Phase I trial revealed a 52% median reduction in serum TTR at a 0.1 mg/kg dosing on day 28, which was below the desired threshold. However, upon completion of a two-year observational phase, all three patients transitioned to a 55 mg dose, reaching a median serum TTR decline of 95% compared to baseline.
Subsequent dosing maintained favorable tolerability profiles. Only one patient experienced a mild infusion-related reaction at the 55 mg dose, indicative of the consistent safety and pharmacokinetics between repeated and single dosing. Even the earliest treated patient demonstrates continued safety and tolerability over three years post-treatment.
Looking Forward
While repeated dosing plans for ATTR amyloidosis are not currently in place for NTLA-2001, these findings mark a significant milestone for Intellia. John Leonard, Ph.D., Intellia’s President and CEO, shared that part of their commitment to patients in the Phase I study was to offer an optimized therapeutic dose if the initial protein reduction was incomplete. The data substantiates the platform’s capability for redosing, opening pathways to treat an array of conditions requiring more than one dose to achieve optimal therapeutic impact.
Comparative Milestones in the Field
Also capturing the spotlight is Alnylam Pharmaceuticals, an RNAi therapeutics pioneer. According to Endpoints News, their latest Phase III clinical trial data for Vutrisiran, treating ATTR amyloidosis with cardiomyopathy, is highly anticipated in 2024. Alnylam’s trials indicate a significant reduction in all-cause mortality rates and success in all secondary endpoints, advancing patient physical function.
Currently, ATTR cardiomyopathy patients have limited options, with Pfizer’s Tafamidis—a TTR stabilizer—being the notable treatment on the market, which garnered impressive sales in the previous year.









































Discussion about this post